Amino acids zwitterions ph 7 ~ What happens to amino acids at high pH. Our group here ensue. Indeed lately has been searched by consumers around us, maybe one of you. People are now accustomed to using the internet in gadgets to see video and image information for inspiration, and according to the title of the post I will discuss about Amino Acids Zwitterions Ph 7 Amino acids with non-ionizable side chains are zwitterions when they are at physiological pH pH 74.
Amino acids zwitterions ph 7 ~ In neutral pH an Amino acids amino group has a postive charge and Carboxyl group has negative charge. They cancel each others charge thanks to the H y d r o g e n thats roaming between the two groups. Your Amino acids zwitterions ph 7 pictures are ready. Amino acids zwitterions ph 7 are a topic that has been hunted for and liked by netizens today. You can Download or bookmark the Amino acids zwitterions ph 7 files here.
Amino acids zwitterions ph 7 | Are All Amino Acids Zwitterions Quora
Amino acids zwitterions ph 7 ~ The neutral zwitterion is the usual form amino acids exist in solution. Amino acids can be linked together the amino group on one amino acid can react with the carboxyl group on another amino acid forming an amide. So for neutral molecules were looking for amino acids that do not carry a charge and the charge group is defined by this. For most amino acids zwitterions would be present at pH 7.
In fact the isoelectric point for many amino acids is about pH 6. The Pka for a carboxyl group is around 3 and the pKa for an amino group is around 9. Amino acids with non-ionizable side chains are zwitterions when they are at physiological pH pH 74. Depending on the pH there are two other forms an anion and a cation.
Explaining why it isnt at pH 7 is quite long-winded and almost certainly beyond what you will need for UK A level or its equivalent purposes. The Pka for a carboxyl group is around 3 and the pKa for an amino group is around 9. If the pH is lower in acidic conditions than the isoelectric point then the amino acid acts as a base and accepts a proton at the amino group. Which is significant as the physiological pH of the cells in our bodies is approximately 74.
Why do amino acids exist as Zwitterions at physiological pH. Because of the charges amino acids are water-soluble. Amino acids with nonionizable side chains are zwitterions when they are at physiological pH pH 74. The Pka for a carboxyl group is around 3 and the pKa for an amino group is around 9.
An amino acid has this ability because at a certain pH value different for each amino acid nearly all the amino acid molecules exist as zwitterions. If the pH is higher in alkaline conditions than the isoelectric point then the amino acid acts as an acid and donates a. If anyone can explain this Id really appreciate it. At physiological pH monoaminomonocarboxylic amino acids eg glycine and alanine exist as zwitterions.
So the isoelectric point should be around 93 2 6 for an amino acid with no ionizable side chain. Most amino acids exist as zwitterions dipolar ions at pH 7. In this problem we wanted list out the amino acids that exist as witter ions at physiological pH values of around 74 but actually exist as neutral molecules. If you are interested the problem is discussed at the bottom of this page.
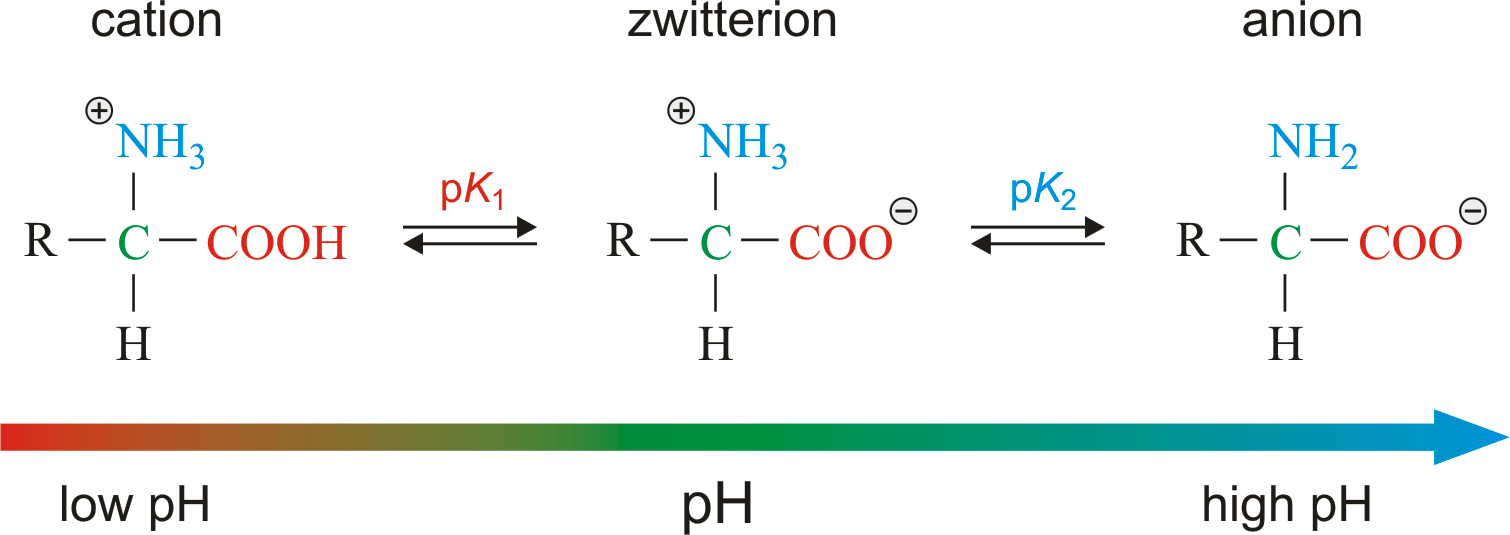
Amino acids with non-ionizable side chains are zwitterions when they are at physiological pH pH. The answer is glutamine but I dont understand why the amino group in glutamines side chain isnt protonated at a pH of 7. The structure of a zwitterion is shown below. With two dissociation steps controlled by two acidity constants K 1 and K 2.
Amino acids with non-ionizable side chains are zwitterions when they are at physiological pH pH 74. So the isoelectric point should be around 93 2 6 for an amino acid with no ionizable side chain. The amino acids in water would have the carboxy group unprotonated and the amino group protonated zwitterion and this is would be fluidly changing. Amino acids with non-ionizable side chains are zwitterions when they are at physiological pH pH 74.
Lysine for example could be represented by the following diagram. This parallels the behavior of a diprotic acid. Are amino acids weak or strong. But I do not understand why.
When calculating the pI of an amino acid that has a titratable group on the R side chain it is useful to start by writing the structure of the amino acid at physiological pH pH 7. At pH values far above or far below 7 its groups can take on different charges - or. The amino acids are Zwitterions. This is what my book says.
Their acidic and basic properties are exceptionally weak for molecules that contain an acid carboxyl group and a basic amino group. Zwitterions form of a compound at neutral pH but it turns out that a zwitterion isnt always a zwitterion. At physiological pH lysine has a net positive charge. Zwitterions AAMC Sample Spoiler In the AAMC Sample FL it asks which amino acid is neutral but a zwitterion at a pH of 7.
That is at a pH of 69-74 the α-carboxyl group pK 24 is dissociated to yield a negatively charged carboxylate ion -COO while the α-amino group pK 97 is protonated to yield an ammonium group NH 3 The pK value of the α-carboxyl group is considerably lower than that of a. When an amino acid dissolves in water the zwitterion interacts with H 2 O molecules acting as both an acid.
If you are searching for Amino Acids Zwitterions Ph 7 you've reached the right location. We have 10 images about amino acids zwitterions ph 7 adding images, pictures, photos, backgrounds, and more. In these web page, we additionally have number of images available. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, translucent, etc.
When an amino acid dissolves in water the zwitterion interacts with H 2 O molecules acting as both an acid. That is at a pH of 69-74 the α-carboxyl group pK 24 is dissociated to yield a negatively charged carboxylate ion -COO while the α-amino group pK 97 is protonated to yield an ammonium group NH 3 The pK value of the α-carboxyl group is considerably lower than that of a. Your Amino acids zwitterions ph 7 pictures are ready in this website. Amino acids zwitterions ph 7 are a topic that has been searched for and liked by netizens now. You can Download or bookmark the Amino acids zwitterions ph 7 files here.
Zwitterions AAMC Sample Spoiler In the AAMC Sample FL it asks which amino acid is neutral but a zwitterion at a pH of 7. At physiological pH lysine has a net positive charge. Your Amino acids zwitterions ph 7 images are ready. Amino acids zwitterions ph 7 are a topic that has been hunted for and liked by netizens today. You can Find and Download or bookmark the Amino acids zwitterions ph 7 files here.
Zwitterions form of a compound at neutral pH but it turns out that a zwitterion isnt always a zwitterion. Their acidic and basic properties are exceptionally weak for molecules that contain an acid carboxyl group and a basic amino group. Your Amino acids zwitterions ph 7 image are available in this site. Amino acids zwitterions ph 7 are a topic that is being hunted for and liked by netizens today. You can Find and Download or bookmark the Amino acids zwitterions ph 7 files here.
This is what my book says. The amino acids are Zwitterions. Your Amino acids zwitterions ph 7 picture are ready. Amino acids zwitterions ph 7 are a topic that has been hunted for and liked by netizens today. You can Find and Download or bookmark the Amino acids zwitterions ph 7 files here.
At pH values far above or far below 7 its groups can take on different charges - or. When calculating the pI of an amino acid that has a titratable group on the R side chain it is useful to start by writing the structure of the amino acid at physiological pH pH 7. Your Amino acids zwitterions ph 7 photographs are ready in this website. Amino acids zwitterions ph 7 are a topic that has been searched for and liked by netizens today. You can Get or bookmark the Amino acids zwitterions ph 7 files here.
But I do not understand why. Are amino acids weak or strong. Your Amino acids zwitterions ph 7 photos are available. Amino acids zwitterions ph 7 are a topic that has been searched for and liked by netizens now. You can Download or bookmark the Amino acids zwitterions ph 7 files here.
This parallels the behavior of a diprotic acid. Lysine for example could be represented by the following diagram. Your Amino acids zwitterions ph 7 picture are ready. Amino acids zwitterions ph 7 are a topic that is being searched for and liked by netizens today. You can Download or bookmark the Amino acids zwitterions ph 7 files here.
Amino acids with non-ionizable side chains are zwitterions when they are at physiological pH pH 74. The amino acids in water would have the carboxy group unprotonated and the amino group protonated zwitterion and this is would be fluidly changing. Your Amino acids zwitterions ph 7 photographs are available. Amino acids zwitterions ph 7 are a topic that has been searched for and liked by netizens today. You can Find and Download or bookmark the Amino acids zwitterions ph 7 files here.
So the isoelectric point should be around 93 2 6 for an amino acid with no ionizable side chain. Amino acids with non-ionizable side chains are zwitterions when they are at physiological pH pH 74. Your Amino acids zwitterions ph 7 images are available. Amino acids zwitterions ph 7 are a topic that is being hunted for and liked by netizens today. You can Find and Download or bookmark the Amino acids zwitterions ph 7 files here.
If the posting of this website is beneficial to your suport by sharing article posts of this site to social media marketing accounts as such as Facebook, Instagram among others or can also bookmark this website page using the title Zwitterionic Structures Of The A Amino Acids Discussed In This Article Download Scientific Diagram Use Ctrl + D for computer system devices with Home windows operating-system or Command word + D for computer devices with operating system from Apple. If you use a smartphone, you can also utilize the drawer menu on the browser you utilize. Whether its a Windows, Apple pc, iOs or Android operating-system, you'll be able to download images using the download button.








0 comments:
Post a Comment